The oxalic acid, Potassium dichromate, and Sulfuric acid (H2C2O4 K2Cr2O7 H2SO4) react with each other. In this reaction, oxalic acid (H2C2O4) reacts with Potassium dichromate (K2Cr2O7). Sulfuric acid creates an acidic environment to help this reaction which is an oxidation-reduction reaction. As we all know oxidation-reduction reactions are known as redox reactions. Since the above reaction is a redox one, we can balance this chemical equation by the ion-electron method.
Oxalic acid, Sulfuric Acid, and Potassium dichromate (H2C2O4 H2SO4 K2Cr2O7) reaction
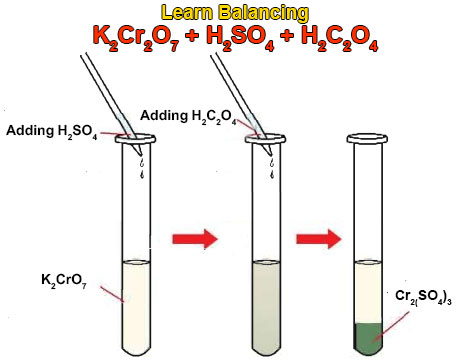
These known chemicals react with each other and produce carbon dioxide gas. This gas will escape away from the reaction mixture. When this redox reaction takes place we will observe a mind-blowing game of color change. The chemical equation for this reaction is given below.
3H2C2O4 + K2Cr2O7 + 4H2SO4 = Cr2(SO4)3 + K2SO4 + 6CO2 + 7H2O
In this oxidation-reduction reaction, the H2C2O4 which is an acid will not act as an acid. Because it is not that strong acid compared to Sulfuric acid. It will act as a reducing agent. On the other hand, K2Cr2O7 will act as the Oxidizing agent.
Reducing agent: H2C2O4 or, C2O4-2, and Oxidizing agent: K2Cr2O7 or, Cr2O7 -2

Reduction Half Reaction
The K2Cr2O7 or, Cr2O7-2 is a strong oxidizing agent so in this reaction, it will behave like an oxidizing agent. In the acidic condition, the potassium dichromate will take up 6 electrons in total so that the oxidation state of Chromium which was +6 on the reactant side will become +3 in the product. So according to this, the reduction half-reaction will take a shape as follow-
Cr2O72- + 6e– + 14H+ = 2Cr3+ + 7H2O … … … (1)
Oxidation Half Reaction
The oxalic acid H2C2O4 is a very weak acid and an organic acid. In the chemical reaction mentioned above, it will act like a reducing agent. It will release two electrons in total and become carbon dioxide. Therefore, the oxidation state of carbon in oxalic acid is +3 and will become +4 after releasing two electrons (one electron each). As per this condition, the oxidation half-reaction takes the shape as follows-
C2O4-2 – 2e = 2CO2 … … … (2)
Balancing the reaction
As we have mentioned above the oxidation half-reaction as well as the reduction half-reaction. Now we can simply add that two half-reactions so that we can get a balanced final reaction. In any oxidation-reduction reaction, the number of electrons donated by the reducing agent and the number of electrons taken up by the oxidizing agent must be the same. Because there is no other way to exchange electrons in the reaction system. Therefore, the reduction half-reaction will multiply 1 time as well as the oxidation half-reaction equation multiplied by 3 times to make the number of electrons equal. Then we add those equations to find a balanced final oxidation-reduction reaction.
Cr2O72- + 6e– + 14H+ = 2Cr3+ + 7H2O
3C2O4-2 – 6e = 6CO2
Cr2O72- + 14H+ + 3C2O4-2 = 2Cr3+ + 7H2O + 6CO2
Now we should add the necessary ions and radicals we get the final reaction.
Cr2O72- + (8H+ + 6H+)+ 3C2O4-2 = 2Cr3+ + 7H2O + 6CO2
K2Cr2O7 + 4H2SO4 + 3H2C2O4 = K2SO4 + Cr2(SO4)3 + 7H2O + 6CO2
Follow us on Twitter, Facebook, Linkedin and Tumbler
Read More

Leave a Reply