Quantum number is the number by which we can identify the position of a particular revolving electron in an atom. In other words, the numbers that defines the size, shape, magnetic orientation in space, and the spin direction of a revolving electron in a particular atom.
How does quantum number determine complete information of electron?
We know the electron is a very tiny little particle in an atom and revolving around the nucleus of the atom. The electrons are revolving outside of the atomic nucleus at a definite energy level. Along with revolving around the atomic nucleus, the electron rotates like the earth as well. On the other hand, we all understand the electron can not be in all the places of an energy level. So the possibility of founding the electron in a very few positions of an energy level is high.

The Orbital concept
The positions in an energy level (orbit) in the atom, where the possibility of finding an electron is very high is known as the orbital. The orbital is a very little space in the energy level or orbit in an atom. The orbital can be spherical, dambal shape, double dambal shape, etc.
The complete information of an electron can be expressed through 4 different quantum numbers. That’s mean the four quantum numbers define the address of an electron in an atom. They are-
- Principal quantum number (n),
- Subsidiary (Azimuthal) quantum number (l),
- Magnetic quantum number (m), and
- Spin quantum number (s).
Now we will briefly discuss those four types of numbers.
Principal Quantum Number (n)
Defines the size of the orbit of the electron that means the energy level in which the electron is present is expressed by this number. It is denoted by the ‘n’. The value of the n could be any positive integer but could not be a fraction. n=1,2,3,4, —–. Besides the energy levels are also denoted by the alphabets starting from K. That means –
- n=1 = K shell
- n= 2 = L shell
- n = 3 = M shell etc.
Subsidiary (Azimuthal) quantum number (l)
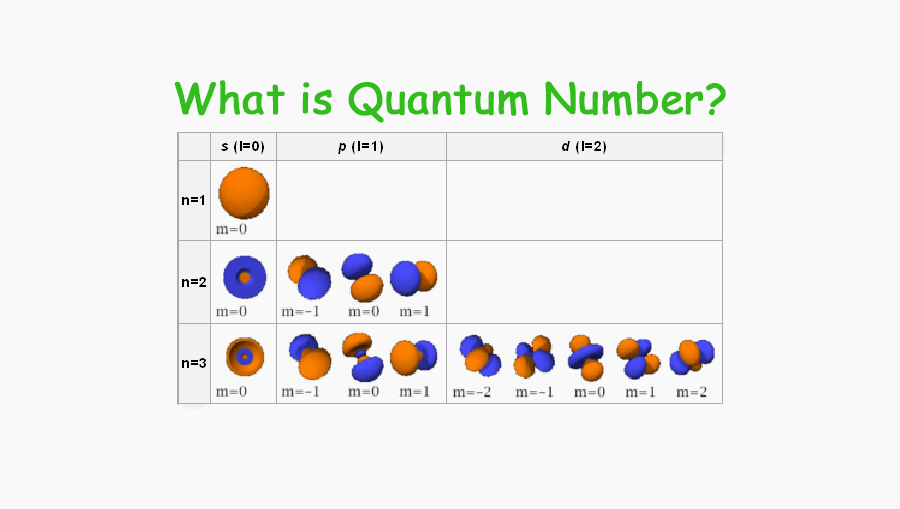
It indicates the shape of an orbital (The positions in an energy level (orbit) in the atom, where the possibility of finding an electron is very high). This number is denoted by the l. The value of “l” depending on the value of “n”. That means the value of “l” is starting from 0 to (n-1).
- If n=1 then l=0
- if n=2 then l=0, and 1
- If n=3 then l=0,1, and 2
Magnetic quantum number (m)
First, you should know that what is the reason for the magnetic word. We all know the electrons revolving around the nucleus and also spinning on its own position like a spinning top.

As we know, if a charged component spins it creates a magnetic field. For these magnetic properties, the electrons can be found in a specific position (that means the orbitals) in a three-dimensional space. So the orbitals are oriented in space in which electron(s) present.
We can define the magnetic quantum number as the quantum number which specifies the orientation of the orbitals of a subshell in three-dimensional space. The magnetic quantum number denoted by “m”.
The value of the “m” is depending upon the value of “l”. The value of “m” can be from -l to +l. That means-
- If l = 0 then m = 0
- If l = 1 then m = -1,0, and +1
- If l = 2 then m = -2,-1,0,+1, and +2
Spin Quantum Number
The fourth number needs to be introduced as the above three numbers were not able to give sufficient explanation about the high-resolution atomic spectra. As we said that the electrons not only revolving around the nucleus but also spinning on its own axis. That means it acts like a thin magnet. This fourth number describes the spinning direction of an electron in the orbital. It denoted by “s”. As the electron can only spin either clockwise or anti-clockwise, this fourth number has only two values.
The value of the “s” is independent of the other three numbers. Instead of giving numbers for s, +1/2, and -1/2 are usually used for the spinning direction of the electrons. These two orientations are usually designated by arrows pointing upwards and downwards (↑↓).
Now, for understanding the numbers lets see the table-
| Principal quantum number (n) | Subsidiary quantum number (l) | Subshell | Magnetic quantum number (m) | Spin quantum number (s) | Number of electrons |
| 3 | 0 | s | 0 | +1/2, -1/2 | 2 |
| 1 | p | -1, 0, +1 | 3(+1/2,-1/2) | 6 | |
| 2 | f | -2, -1, 0, +1, +2 | 5(+1/2, -1/2) | 10 |
Follow us on Twitter, Facebook, Linkedin
Read more Oxidation-reduction (Redox) reactions

Leave a Reply