Adsorption of acetic acid on charcoal and validity of Freundlich’s adsorption isotherm and Langmuir’s adsorption isotherm
Adsorption of acetic acid on charcoal is the topic of discus for today. Adhesion to a surface is called the adsorption of atoms, ions, biomolecules, or gas, liquid, or dissolved solids molecules. The activated charcoal adsorbs acetic acid. The activated charcoal adsorbs the acids in their porous meanwhile this process follows the adsorption isotherm of Freundlich as well as isotherm of Langmuir. Adsorption of acetic acid on activated charcoal is a very important topic to understand the validity of Freundlich’s adsorption isotherm as well as Langmuir’s adsorption isotherm.
The amount adsorbed by an adsorbent is dependent on pressure and temperature. Thus the amount (X) adsorbed is a function of pressure (P) and temperature (T), i.e.
X= f(P,T)
A plot of P vs X, keeping temperature constant is known as adsorption isotherm.
Freundlich’s adsorption isotherm:
Freundlich’s adsorption isotherm; represented by –
X/m = kc1/n
where m= the amount of adsorbing material C is the equilibrium constant of adsorbate in the solution k is the constant depending on the nature of both adsorbate and adsorbant n is another constant depending on the nature of both adsorbates.
The value of 1/n is less than unity. On taking the logarithm of the equation,
log (x/m)= 1/n log c + log k —————————— (2)
If a plot of log (x/m) vs log c gives a straight line with a slope of 1/n and intercept of log k, Freundlich’s adsorption isotherm is valid
Langmuir’s adsorption isotherm:
According to Langmuir for a uni-molecular layer, the following relation holds for adsorption,
x/m = k1k2c/(1+k1c)
where k1 and k2 are the constant.
The above equation can be written as-
c/(x/m) = 1/ k1k2 + c/k2
Thus a plot of c/(x/m) vs c gives a straight line with a slope of 1/k2 and an intercept of 1/ k1k2 and shows the validity of Langmuir adsorption isotherm
Apparatus and chemicals:
Burette, pipette, Activated charcoal, 0.5N acetic acid, 0.1N NaOH, Stopped bottles, Shaking machine, etc.
Procedure:
- Prepare 0.5N acetic acid as well as 0.1N NaOH solution.
- Take 6 clean and dry stopped bottles and prepare the following solutions in the table-
| Bottle No | 0.5N Acetic acid(mL) | Distilled water (mL) | Amount of charcoal (g) |
| 01. | 50 | 0 | 1 |
| 02. | 40 | 10 | 1 |
| 03. | 30 | 20 | 1 |
| 04. | 25 | 25 | 1 |
| 05. | 20 | 30 | 1 |
| 06. | 10 | 40 | 1 |
- Spotted each bottle after that shake for at least 1 hour.
- Filter the solution through filter paper as well as collect the filtrate in the numbered bottle. reject the first 5 mL of each filtrate.
- Pipette out 10 mL of each filtrate and titrate with 0.1N NaOH solution.
- Note Down the temperature of the solution.
Data :
Bottle No: 01
| Activated solution (mL) | IBR | FBR | BR difference | mean |
| 10 | 0 | a1 | a1 | let the mean be x1 |
| 10 | a1 | b1 | a1-b1 | |
| 10 | b1 | c1 | b1-c1 |
Bottle No: 02
| Activated solution (mL) | IBR | FBR | BR difference | mean |
| 10 | 0 | a2 | a2 | let the mean be x2 |
| 10 | a2 | b2 | a2-b2 | |
| 10 | b2 | c2 | b2-c2 |
Bottle No: 03
| Activated solution (mL) | IBR | FBR | BR difference | mean |
| 10 | 0 | a3 | a3 | let the mean be x3 |
| 10 | a3 | b3 | a3-b3 | |
| 10 | b3 | c3 | b3-c3 |
Bottle No: 04
| Activated solution (mL) | IBR | FBR | BR difference | mean |
| 10 | 0 | a4 | a4 | let the mean be x4 |
| 10 | a4 | b4 | a4-b4 | |
| 10 | b4 | c4 | b4-c4 |
Bottle No: 05
| Activated solution (mL) | IBR | FBR | BR difference | mean |
| 10 | 0 | a5 | a5 | let the mean be x5 |
| 10 | a5 | b5 | a5-b5 | |
| 10 | b5 | c5 | b5-c5 |
Bottle No: 06
| ActivatedActivaated solution (mL) | IBR | FBR | BR difference | mean |
| 10 | 0 | a6 | a6 | let the mean be x6 |
| 10 | a6 | b6 | a6-b6 | |
| 10 | b6 | c6 | b6-c6 |
Calculation:
| Bottle No | Initial conc. of the acetic acid before adsorption (C0) | Equilibrium conc. of the acetic acid before adsorption (Ce) | Amount of acetic acid adsorped (C0-Ce) |
| 01 | 50 | x1 | y1 |
| 02 | 40 | x2 | y2 |
| 03 | 30 | x3 | y3 |
| 04 | 25 | x4 | y4 |
| 05 | 20 | x5 | y5 |
| 06 | 10 | x6 | y6 |
Table for the proof of Freundlich’s adsorption isotherm
| Bottle No. | Amount of acetic acid adsorped (C0-Ce) = (x/m) | log(x/m) | Ce= C | log C |
| 01 | y1 | log y1 | x1 | log x1 |
| 02 | y2 | log y2 | x2 | log x2 |
| 03 | y3 | log y3 | x3 | log x3 |
| 04 | y4 | log y4 | x4 | log x4 |
| 05 | y5 | log y5 | x5 | log x5 |
| 06 | y6 | log y6 | x6 | log x6 |

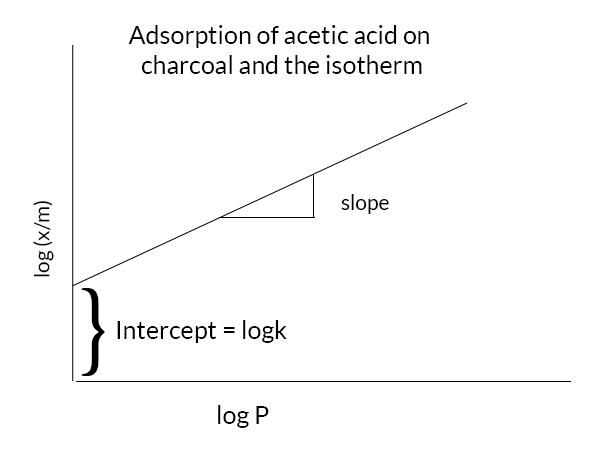
Freundlich’s adsorption equation
log(x/m) = logk +1/n logC
from the above graph, we can see that the graph is a straight line and the slope is 1/n the intercept is log k. we can find the value of k from the intercept.
Table for the plot of Langmuir adsorption isotherm:
| Bottle No. | Equilibrium conc. of the acetic acid before adsorption (Ce) | Amount of acetic acid adsorped (C0-Ce) = (x/m) | Ce /(x/m) |
| 01 | x1 | y1 | x1/y1 |
| 02 | x2 | y2 | x2/y2 |
| 03 | x3 | y3 | x3/y3 |
| 04 | x4 | y4 | x4/y4 |
| 05 | x5 | y5 | x5/y5 |
| 06 | x6 | y6 | x6/y6 |

From the Langmuir equation
Ce/(x/m) = 1/(k1k2) + c/k2
from the above graph, we can see that the graph is a straight line and the slope is 1/k2 the intercept is 1/(k1k2). we can find the value of k2 from the slop. After that, we can also find the k1 from the intercept and the value of k2.
The concentration of acetic acid after adsorption:
- The initial concentration of acetic acid is = 0.50N
- Calculation of the equilibrium concentration of acetic acid:
| we know, VaSa = VbSb
Sa=VbSb/Va |
here,
|
| Bottle No. | Sa |
| 01 | S1 |
| 02 | S2 |
| 03 | S3 |
| 04 | S4 |
| 05 | S5 |
| 06 | S6 |
Result:
- The validity of Freundlich’s adsorption isotherm is proved from the above first plot.
- The validity of the Langmuir adsorption isotherm is proved from the above second plot.
Follow Us On Facebook, Twitter, Linkedin, and Tumbler.
Read more

Leave a Reply