
Balancing K2Cr2O7 + FeCl2 + HCl = KCl + CrCl3 + FeCl3 + H2O
K2Cr2O7, FeCl2, and HCl are very common chemicals in all most every laboratory in the world. Besides, all the science students, as well as the Read More

K2Cr2O7, FeCl2, and HCl are very common chemicals in all most every laboratory in the world. Besides, all the science students, as well as the Read More

KMnO4, HCl, and FeCl2 are the reactants of the above reaction. But we have to know that the HCl in this reaction is creating the Read More
K2Cr2O7, FeSO4, and HCl are the reactants of the above reaction. Mainly, K2Cr2O7 and FeSO4 react with each other, while they exchange the electrons. Yes, K2Cr2O7 Read More
The Sodium sulfite reacts with potassium permanganate and sulfuric acid. Sodium sulfite, potassium permanganate, and sulfuric acid (KMnO4 H2SO4 Na2SO3) are very common and well-known Read More
Calcium oxalate, Sulfuric Acid, and Potassium Permanganate (CaSO4 H2SO4 KMnO4) are very common chemicals all around the world. These three chemicals react with each other. Read More
Very common chemicals are Potassium Dichromate, Hydrogen Sulfide and Sulfuric Acid (K2Cr2O7 H2S H2SO4). They respond to the formation of sulfur, potassium sulfate, chromium sulfate, Read More
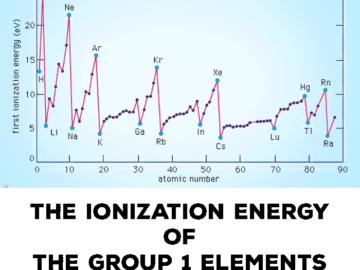
periodic table ionization energy (I.E.) is an important property for the elements. It helps to understand the affinity of an element for electrons as well Read More

When the Sulfuric acid is diluted, the Aluminium reacts with the diluted Sulfuric acid (Al H2SO4) to produce Aluminium sulfate as well as Hydrogen gas. The reaction Read More

Aluminum (Al H2SO4) reacts with the concentrated sulfuric acid to generate aluminum sulfate Sulfer di-oxide as well as water when the sulfuric acid is very Read More
Hydrogen sulphide, potassium permanganate, and sulfuric acid (H2S KMnO4 H2SO4) are available in almost all chemical labs across the World. In the H2SO4 medium, KMnO4 Read More