Liquid-Liquid Miscibility of three liquid systems and position of one tie line
The liquid-liquid miscibility of three liquid systems is based on the phase rules. Knowing the mutual solubility of liquids in a two-phase scheme is sometimes essential. For example, you may need to know how much water is dissolved in an organic liquid substance with which it is in contact, as well as the amount of the organic compound that is in the aqueous phase, with which water is in contact. In this experiment, a three-component mixture (Chloroform – water – 25oC acetic acid and barometric pressure) will be considered and the appropriate ternary phase diagram will be constructed.
According to the face rule, a single face in the three-component system (Chloroform – water – acetic acid at 25 oC and barometric pressure) may possess four degrees of freedom.
F= C-P+2 ——————–(1)
where F= degree of freedom. C = component. P= Phase
These are temperature, pressure, and the compositions of the two of the three components. Because of the difficulty in graphically so many variables, temperature and pressure are generally held constant, so the face rule reduced to
F=C-P=3-P—————-(2)
Do you get it that, this is the same special form of the face rule that is applied to the two-component system (e.g. one of the liquid is A and another one is B) of constant pressure only?
Graphical Representation of the Three-Component system:
For the three-component system, the properties of the equilateral triangle provide a convenient means of representing the composition of a three-component system at constant temperature and pressure. Line AB represents the compositions of all the systems that can be prepared from pure A as well as B. At point P the system is 60%B and 40% A.

Chemicals and Apparatus
Five Stopped bottles, burette, Acetic acid, chloroform, water, etc.
Procedure of miscibility of three liquid systems
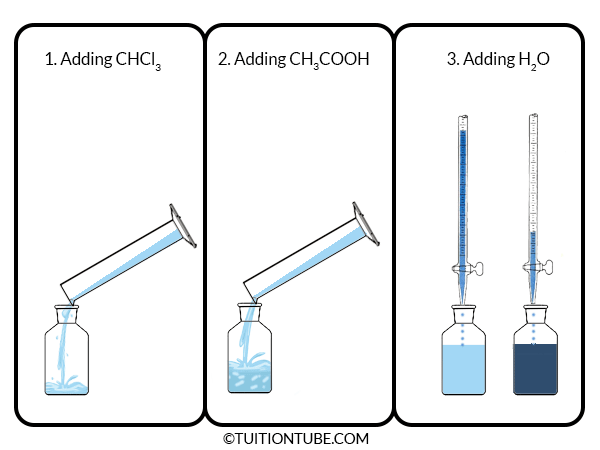
- Make 20 g of CHCl3 and CH3COOH mixture of varying composition in nine different stopped bottles with the weight of 10, 20, 30, 40, 50, 60, 70, 80, 90% CHCl3. The density of CHCl3 and CH3COOH are 1.50 and 1.05 respectively.
- Now add water into the solution from the burette with continuous shaking until the turbidity appears in the stopped bottles.
- From the amount of water added and the initial amount of CHCl3 and CH3COOH, the percentage of composition of the mixture is calculated as well as the results are plotted in a triangle diagram.
- Note the room temperature.
Data for liquid-liquid miscibility of three liquid system
| No. of Observation | the volume of chloroform (mL) | the volume of acetic acid (mL) | IBR | FBR | the volume of water needed to obtain turbidity (mL) |
| 1 | a1 | b1 | p1 | q1 | c1 |
| 2 | a2 | b2 | p2 | q2 | c2 |
| 3 | a3 | b3 | p3 | q3 | c3 |
| 4 | a4 | b4 | p4 | q4 | c4 |
| 5 | a5 | b5 | p5 | q5 | c5 |
| 6 | a6 | b6 | p6 | q6 | c6 |
| 7 | a7 | b7 | p7 | q7 | c7 |
| 8 | a8 | b8 | p8 | q8 | c8 |
| 9 | a9 | b9 | p9 | q9 | c9 |
Calculation:
From the above information, we can draw the following graph.

Result of liquid-liquid miscibility of three liquid systems
Curve obtained from the joining experimental determined point from the boundary between the homogenous and heterogeneous mixture. From the tie line represented in the graph, the composition of the liquid system can be found.
Follow Us On Facebook, Twitter, Linkedin, and Tumblr.
Read more about-

Leave a Reply