The cis-tans isomers are very common among a very large number of substances in organic chemistry. Sometimes, It becomes so much important to know that if a compound taken under concern is a cis or a trans isomer. To know perfectly cis or trans isomer, physical methods are very easy and fast processes.
Physical methods for the determination of cis-trans isomers
To determine the cis-trans isomer of a substance following physical methods are used. The methods are-
- Vibrational (IR-Raman) spectra
- NMR ( 1H, 13C both)
- UV- visible spectra
- Refractive index
- Density
- Boiling points
- Dipole moments
- Less often mass spectra
- Also X-ray, electron diffraction method, as well as microwave spectra.
Other physical methods such as Density, refractive index, boiling point, melting point, etc are not very reliable to determine the cis-trans isomers.
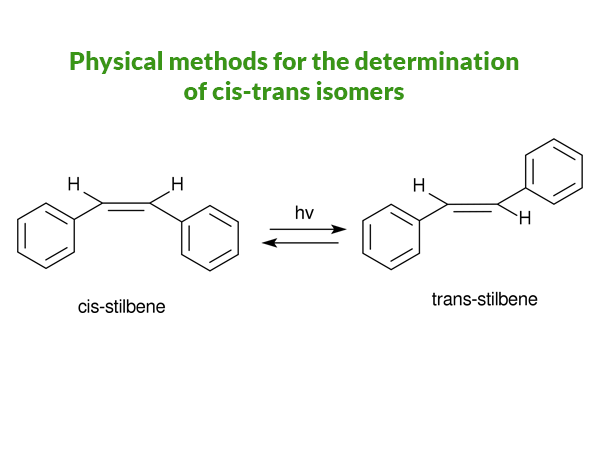
UV-visible spectra for Determination of Cis-trans Isomer
Normally stabilization of the ground ( trans-stilbene is more stable than its cis-counterpart: π–overlapping in coplanar trans-stilbene makes it more stable, o-hydrogens interact to force out (430) the benzene ring of the plane in cis-stilbene. No such π- overlapping so less stable) state shifts λ max to
- shorter λ whereas stabilization of the excited state shifts λ max to longer wavelength.
- Still, the more stable trans isomer absorbs at the longer wavelength

The singlet excited state is a diradical and since the Franck-Condon principle demands that it have the same geometry as the ground state, stabilization of the radical on the side of the twisted benzene ring by benzylic resonance is sterically impeded, thus explaining the much higher energy level of the singlet excited state of the cis isomer.
Though UV-visible spectrum is used to determine the cis-trans isomer in some instants as the physical method this is not a very good physical method that can be treated without a doubt.
Vibrational (IR-Raman) spectra for Determination of Cis-trans Isomer
Vibrational spectra such as Infrared rays i.e. IR spectra or Raman spectra can be used in some cases to determine the cis-trans isomers with confidence. For example, trans-1,2-dichloroethylene, (E) –CHCl = CHCl. This particular compound shows no IR spectrum during vibration. This means it does not absorb any IR at all. Because the dipole moment of the trans-1,2-dichloroethylene is zero and no change in μ during vibration. Therefore, no IR absorption occurs. On the other hand, there is a strong Raman absorption found at 1577 cm-1 in the case of trans-1,2-dichloroethylene. Because of change in polarizability of trans-1,2-dichloroethylene, (E) –CHCl = CHCl.
But when we consider the IR spectrum of cis-1,2-dichloroethylene, (Z) –CHCl=CHCl. We get a strong IR absorption for C=C stretching vibration at 1590cm-1. Thus we can determine if the compound taken under concern is a cis or a trans isomer.
NMR spectroscopy for Determination of Cis-trans Isomer
NMR is by far the most useful & versatile technique for distinguishing cis and trans isomer. NMR means Nuclear magnetic resonance. It is a very advanced technique where all the Hydrogen atoms present in a molecule can be determined as well as the number of the C atoms in the molecule can also be determined. But the hydrogens in a molecule with the same chemical environment show single and unique peaks in the NMR graph. But it is an interesting fact that the intercity of the peak or peaks become strong as the number of the Hydrogen increases.
In both 1H and 13C NMR spectrum the chemical shifts and coupling constants are useful to determine the cis-trans isomers.
In alkenes of the type RCH = CHR” or RCH = CFR’; RCF=CFR’; H-H, H-F, F-F coupling constants can be used to assign configuration.
Mass spectrometry is not a major tool for distinguishing cis-trans isomers
Chromatographic methods do not usually serve to identify cis-trans isomers.

Leave a Reply