The standard electrode potential of copper and zinc is an important experiment worldwide. For general chemistry as well as applied chemistry, this experiment is worthwhile. Concerning a standard hydrogen electrode (SHE), values for standard electrode potentials are most often tabulated at 25 ° C. It is also an electron exchange reaction which means a Redox reaction.
Determination of the standard electrode potential of copper and zinc:
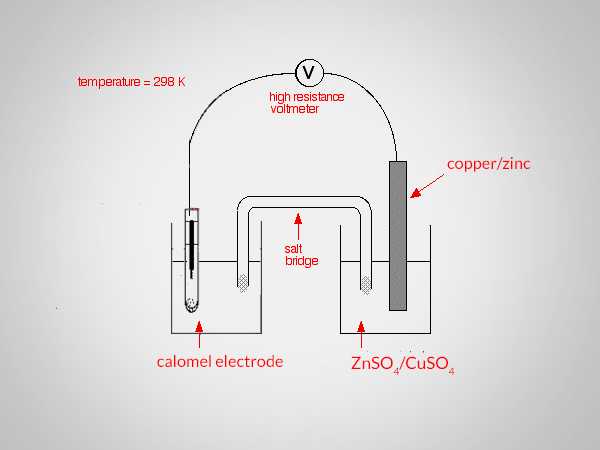
To determine the standard electrode potential of copper and zinc the standard calomel electrode (SCE) is connected with a copper or zinc electrode and the emf of the cell is determined using a potentiometer,
Ecell = Ecathode(red) – Eanode(red)
The zinc cell (Zn) could be portrayed as
Zn/Zn2+ || Calomel (standard)
The Copper cell (Cu) could be portrayed as
Calomel (standard) || Cu2+/Cu
From the Nurst equation –
E=E0 – (2.303RTloga)/nF
Apparatus as well as chemicals required:
- Potentiometer
- standard cell
- Galvanometer
- Standard Calomel electrode
- Zinc electrode
- Copper electrode
- 0.1M Solution of ZnSO4 and CuSO4
Procedure:
- 0.0001M, 0.001M and 0.01M solution is prepared.
- Then with the help of the calomel electrode and Potentiometer, we determine the potential of the Zn electrode at each concentration.
- For cupper, the above process should be repeated.
Example Data of the Experiment:
emf of the ZnSO4 solution
| Concentration of ZnSO4 | emf |
| 0.1 | a |
| 0.01 | b |
| 0.001 | c |
| 0.0001 | d |
emf for CuSO4 solution
| Concentration of ZnSO4 | emf |
| 0.1 | e |
| 0.01 | f |
| 0.001 | g |
| 0.0001 | h |
Calculation:
Ecell of Cu electrode
we will use the Nurst equation to determine the standard emf of the copper as well as zinc.
| concentration | emf | mean activity coefficient | MCu2+. γ+ | log(MCu2+xγ+) |
| 0.0001 | h | 1.00 | 0.0001 | -4 |
| 0.001 | g | 0.734 | 0.00734 | -3.14 |
| 0.01 | f | 0.4 | 0.004 | -2.397 |
| 0.1 | e | 0.161 | 0.0161 | 1.79 |
Ecell of Zn electrode
The Nurst equation will be used to determine both the normal zinc emf and copper emf.
| concentration | emf | mean activity coefficient | MCu2+. γ+ | log(MCu2+xγ+) |
| 0.0001 | d | 1.00 | 0.0001 | -4 |
| 0.001 | c | 0.734 | 0.00734 | -3.14 |
| 0.01 | b | 0.4 | 0.004 | -2.397 |
| 0.1 | a | 0.161 | 0.0161 | 1.79 |
Sample of Graphs:


Result:
From the above Experiment to determine the standard electrode potential of copper as well as zinc we get the two graphs.
Now From the graphs,
“The standard electrode potential of copper and zinc would be 0.084 volts and 1.06 volts respectively.”
Follow us on Twitter, Facebook, Linkedin
Read More:
K2Cr2O7 + FeSO4 + H2SO4 = Cr2(SO4)3 + Fe2(SO4)3 + K2SO4 + H2O

Leave a Reply