
KMnO4 + H2O2 + H2SO4 = O2 + MnSO4 + K2SO4 + H2O
Potassium permanent hydrogen peroxide and sulfuric acid (kmno4 h2o2 h2so4) are accessible in nearly all chemical laboratories around the world. KMnO4 and H2O2 react mainly Read More

Potassium permanent hydrogen peroxide and sulfuric acid (kmno4 h2o2 h2so4) are accessible in nearly all chemical laboratories around the world. KMnO4 and H2O2 react mainly Read More

We’re learning today- what redox reaction is as well as examples of it. Redox (reduction-oxidation reaction) is a chemical reaction that occurs by exchanging electrons, atom Read More
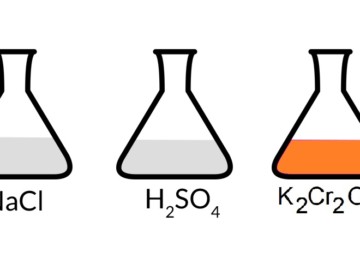
Potassium dichromate, iron(II) sulfate, and sulfuric acid (K2Cr2O7 FeSO4 H2SO4) are accessible in nearly every chemical laboratory worldwide. In the acidic medium, K2Cr2O7 and FeSO4 Read More

Iron(II) sulfate where Iron has an oxidation number of +2 and Potassium permanganate where Mn has an oxidation number of +7, react with each other Read More

Sodium chloride, Potassium permanganate react with each other in an acidic environment (NaCl KMnO4 H2SO4) to produce the free chlorine. This is a Redox reaction. Read More

How To Draw A Dog is a common topic in schools. In schools we learn to draw and drawing a dog is a simple topic. Read More

All the public living the modern world must know something about the vitamins, which are essential organic substances needed in a small amount for the Read More

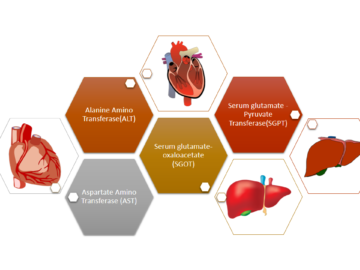
When someone becomes sick and visits a doctor, the doctor first tries to identify the cause of the disease. Luckily if the doctor can find Read More
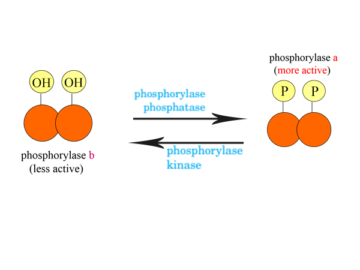
Phosphorylation is the most important process for plants as well as animals i.e. for the whole world. This is the process in which plants convert the Read More